What Is the PREP Act?
There would be no COVID mRNA vaccines without it, yet few know it even exists.

In conjunction with EUA (Emergency Use Authorization), the PREP Act is the legislation that enabled – and continues to perpetuate – the rollout and administration of mRNA “countermeasures” against Covid-19.
In this article I will discuss what the PREP Act says, how it was passed, what prominent politicians and legal experts said about it at the time, how it is related to COVID, and why I support efforts (1) calling for the HHS Secretary to immediately repeal the PREP Act emergency declaration for COVID, and (2) calling on legislators to repeal the law entirely.
WHAT THE PREP ACT SAYS
The PREP Act is a long and convoluted piece of legislation. You can read the entire thing here:
This is a summary of the main sections of the law:
(a) Liability Protections
Anyone defined as a “covered person” is immune from legal liability related to the use or administration of anything defined as a “covered countermeasure.”
A “covered person” includes (A) “the United States” or (B) any person or “entity” that manufactures, distributes, plans a program for, prescribes, administers, or dispenses a covered countermeasure, or an official, agent or employee of any of the above.
A “covered countermeasure” includes any drug, biological product or device that is authorized under Emergency Use Authorization or approved through any other legal pathway.
Scope of claims for loss:
The immunity applies to any claim related to death, actual or fear of physical, mental or emotional injury, illness, disability, or condition; and loss of or damage to property, including business interruption loss.
The immunity applies to any causal relation to any of the above types of loss related to the design, development, clinical testing or investigation, manufacture, labeling, distribution, formulation, packaging, marketing, promotion, sale, purchase, donation, dispensing, prescribing, administration, licensing, or use of a covered countermeasure.
The immunity applies only if a countermeasure was applied or used during the effective period of the emergency declaration for that countermeasure, and was used for the disease, population and geographic area specified in the declaration.
For manufacturers or distributors, the immunity applies to any population in any geographic area, without regard to the population or area specified in the emergency declaration for the countermeasure.
(b) Declaration by Secretary
The HHS Secretary has the sole discretion to determine that a disease or other health condition or other threat to health constitutes a public health emergency, or that there is a credible risk for a future such emergency and, based on that determination, to make a declaration recommending the manufacture, testing, development, distribution, administration or use of one or more covered countermeasures, thereby activating the legal immunity described in section (a).
In the emergency declaration, which is made by publishing it in the Federal Register, the secretary shall identify – with respect to the use of countermeasures – the category of threat, the period during which the threat is in effect, the population for which it is in effect, and the geographic area for which it is in effect.
The period during which the emergency declaration is effective is flexible, depending on various determinations by the Secretary.
The Secretary can change any aspect of the declaration of emergency without retroactively affecting the immunity granted under the declaration.
The Secretary’s decision to issue an emergency declaration for immunity can be based on anything, including the “desirability of encouraging” the design, development, clinical testing or investigation, manufacture, labeling, distribution, formulation, packaging, marketing, promotion, sale, purchase, donation, dispensing, prescribing, administration, or licensing of a covered countermeasures
No court – whether Federal or State – has subject matter jurisdiction to review any action by the Secretary related to the emergency declaration
No State may pass or enforce any law that is different from or in conflict with anything related to the declaration of emergency or to anything related to the qualified persons or covered countermeasures.
(c) Definition of Willful Misconduct
(d) Exception to Immunity of Covered Persons
These two sections define the circumstances under which the PREP Act immunity does not apply. In general, the sole exception is defined as “an exclusive Federal cause of action against a covered person for death or serious physical injury caused by willful misconduct.”
The definition of “willful misconduct” is: “an act or omission taken intentionally to achieve a wrongful purpose; knowingly without legal or factual justification; and in disregard of a known or obvious risk that is so great as to make it highly probable that the harm will outweigh the benefit.” This is specifically defined as “a standard for liability that is more stringent than a standard of negligence in any form or recklessness.”
A plaintiff who tries to sue under this section has “the burden of proving by clear and convincing evidence willful misconduct.”
Exceptions to “willful misconduct”
7-day disclosure exception: If a qualified person or program planner acted under the HHS Secretary's directions, guidelines or recommendations regarding a covered countermeasure, and if that person or planner provided within seven days “either the Secretary or a State or local health authority” with “notice of information” related to a plaintiff’s alleged loss, then that person or planner “shall not have engaged in ‘willful misconduct’ as a matter of law.”
Lack of Federal action against a manufacturer/distributor exception: If neither the HHS Secretary nor the Attorney General initiates enforcement action with respect to an act or omission in a willful misconduct claim against a manufacturer or distributor, then that act or omission “shall not constitute ‘willful misconduct.’”
(e) Procedures for Suit
Any willful misconduct action can only be filed in the U.S. District Court for the District of Columbia. In adjudicating the action, Federal law preempts any State law in which the alleged willful misconduct occurred.
SUMMARY:
The Secretary of Health and Human Services has the authority to declare a public health emergency based on any present or future risk determined at his sole discretion, to apply that emergency to any period of time, any population and any geographic area. Once he declares the emergency, he can recommend the manufacture and distribution of any countermeasures, again based on his sole discretion. Anyone who does anything with those countermeasures is immune from any legal liability. Courts cannot make any rulings regarding the legality of declarations or activities covered by this Act. No State law can supersede this act.
HOW THE PREP ACT WAS PASSED
The extremely consequential and arguably unconstitutional PREP Act became part of U.S. law in December 2005 through a surreptitious, rushed process that allowed little to no debate or discussion. Here’s how it happened: [ref][ref][ref]
-Sunday, December 18, 2005, the US House and Senate finished their deliberations over the FY2006 budget, an omnibus spending bill.
-Night of Sunday, December 18, 2005, the PREP Act was inserted into the defense appropriations bill, which was part of the omnibus spending bill.
-Monday, December 19, 2005, the appropriations bill with the PREP Act legislation was approved by the House of Representatives in a vote of 308–106. According to the Clerk’s records, the vote took place at 5:04AM (which would be just a few hours after the PREP Act had been inserted).
-Tuesday, December 20, 2005, law professor and legal scholar Erwin Chemerinsky submitted a letter to Sen. Patrick Leahy detailing ways in which the PREP Act is unconstitutional.
-Wednesday December 21, 2005, The Chemerinsky letter was entered into the Congressional Records, and the only debate/discussion about the PREP Act took place. Members of the Senate expressed their frustration with the fact that the Act was inserted secretly into the spending bill with no time for debate and no committee reviews or discussions. They raised many objections, challenging the Constitutionality of the law. [see below]
-Thursday December 22, 2005, despite those vehement objections, the Senate passed the omnibus bill with the PREP Act, 93-0 (7 not voting) [ref] and then Congress adjourned for the year.
OBJECTIONS TO THE PREP ACT THE DAY BEFORE IT WAS PASSED
On December 21, 2005 – the only day on which the PREP Act was ever discussed or debated by any legislator in either chamber of Congress – a number of prominent Senators raised very serious objections to the proposed legislation (which, as they pointed out, is not an appropriation but an actual law) and the way in which it was snuck into the appropriations bill. Objections notwithstanding, the following day they voted to pass the appropriations bill that included the PREP Act – which then became law.
Here are some of the Senators’ objections to the PREP ACT: [ref][ref]
Sen. TED KENNEDY:
I ask unanimous consent to amend the resolution to strike division E, the Public Readiness and Emergency Preparedness Act. This is the provision that provides drug companies with unprecedented immunity from liability which was added to the Defense appropriations bill in the conference during the middle of the night. It does not belong in this bill.
With regard to the manufacturers? What kind of immunity have we given to them? It’s really extraordinarily broad, effectively complete.…So companies are not deterred from acting recklessly, or with gross negligence.
The bill defines a very narrow standard of willful misconduct, and it sets a very high standard of evidence. Shouldn’t that be enough? Wrong. You don’t have a case against a company under these provisions unless the FDA begins an enforcement case against that company. So if FDA goes ahead and begins the case, you have a chance, right? Wrong again. FDA has to bring it and conclude it successfully before you have any right to proceed with your case.
A person might think, I am not very satisfied with how this liability provision has worked, maybe I will appeal to the courts of this country, right? Wrong. There is absolutely no, no, no, no judicial review when the Secretary of Health and Human Services grants a company immunity by issuing a declaration. No judicial review of that. And there is no judicial review of FDA’s decision not to bring an enforcement action. So it is whatever the administration says, whatever the Secretary says, whatever the head of the FDA says, with changed and gimmick rules.
This is a sham. There is no possibility of liability here.
Slipping a provision into a major spending bill late at night at the end of [sic] Congressional session is a trick to shield from public debate a provision that is so wrongheaded that it would never stand public scrutiny.
Sen. JOE BIDEN:
I rise to express my surprise and deep-seated opposition to the so-called Public Readiness and Emergency Preparedness Act, which is included in the Defense Department Appropriations bill. This provision would give the Secretary of Health and Human Services authority to provide almost total immunity from liability to the makers of almost any drug, and to those who administer it. While the measure’s proponents portray it as a simple tool to make sure we have sufficient vaccine [sic] available in the case of an avian flu pandemic, the actual language of the provision is far broader than that, and it therefore poses a danger to all Americans.
The actual provision permits immunity for the makers of virtually any drug or medical treatment. All the secretary need do is declare that it is a ‘‘countermeasure’’ used to fight an epidemic. One solitary person gets to decide what is a countermeasure and what is an epidemic. There is nothing to prevent the declaration of immunity for, say, Tylenol. There is nothing to prevent a declaration that, say, arthritis is an epidemic.
What’s more, this is no typical grant of immunity. No, the breadth of this provision is staggering. A drug maker can be grossly negligent in making or distributing a drug, and still escape liability. It can even make that drug with wanton recklessness and escape scott-free after harming thousands of people.
No hearings were held on this language; no Committee vote was taken; no bill passed the House or the Senate. Not even the House and Senate conferees had a chance to give input on this provision.
Sen. HILLARY CLINTON:
I support limited liability protections for manufacturers to help cover their risks in developing products that our Nation will need in case of emergency. However, this provision would grant immunity to all claims of loss, including death and disability, for a broad range of products, including any drug that the Secretary designated as one that would limit the harm caused by a pandemic—a definition so broad as to encompass nearly any drug. This immunity is not subject to judicial review. It preempts any State laws that provide different liability protections or that may provide stronger consumer safety protections for pharmaceutical products.
This authorizing—authorizing, not appropriating—language was never considered, let alone agreed to by the Senate. It was never agreed to by the HELP or Judiciary Committees, which have jurisdiction over this matter. It is a mockery of the legislative process. I believe that the American people are ill-served by Congress when controversial and potentially harmful provisions can simply be inserted without undergoing the open deliberations and debate that are fundamental to the democratic process and are designed to protect our citizens from special interests and back-room dealings.
Sen. ROBERT BYRD:
I continue to have serious concerns about the avian flu-related liability provisions that were slipped into the conference report without debate. These liability provisions did not appear in either the House- or the Senate-passed bill. These provisions were not in the materials presented to the conference committee during its deliberations. It was not until the dead of night on this past Sunday, after signatures had already been collected on the conference report, that the Republican majority slipped these provisions into the bill before the Senate today. What an insult to the legislative process.
Sen. PATRICK LEAHY:
This provision is a gift to the drug manufacturers and will likely have a devastating effect on our ability to protect our constituents. Under the guise of a threatened pandemic, this legislation goes far beyond the scope of vaccine preparedness and includes language that is far more sweeping than any language previously passed by the House or the Senate.
The only exception to the broad immunity given to drug companies in this proposal is the possibility that a victim could prove that the company acted with ‘‘willful misconduct.’’ Knowingly committing health violations would not even suffice to state a claim. Knowing violations as well as gross negligence would be immunized from accountability. Even if the drug company acted with the intent to harm people, it would nevertheless be immune from criminal conduct unless the Attorney General or Secretary of Health and Human Services initiates an enforcement action against a drug company that is still pending at the time a personal claim is filed. That is unbelievable.
I question whether such a role for the Secretary of HHS is even constitutional. Since when do we in Congress allow a political appointee of the administration to determine when, and if, someone injured by willful misconduct can be compensated for their injuries?
Passage of the Defense appropriations bill is of vital importance to all of us, but the inclusion of provisions that excuse even gross and deadly negligence on the part of drug companies makes it impossible for many of us to vote for this bill in good conscience.
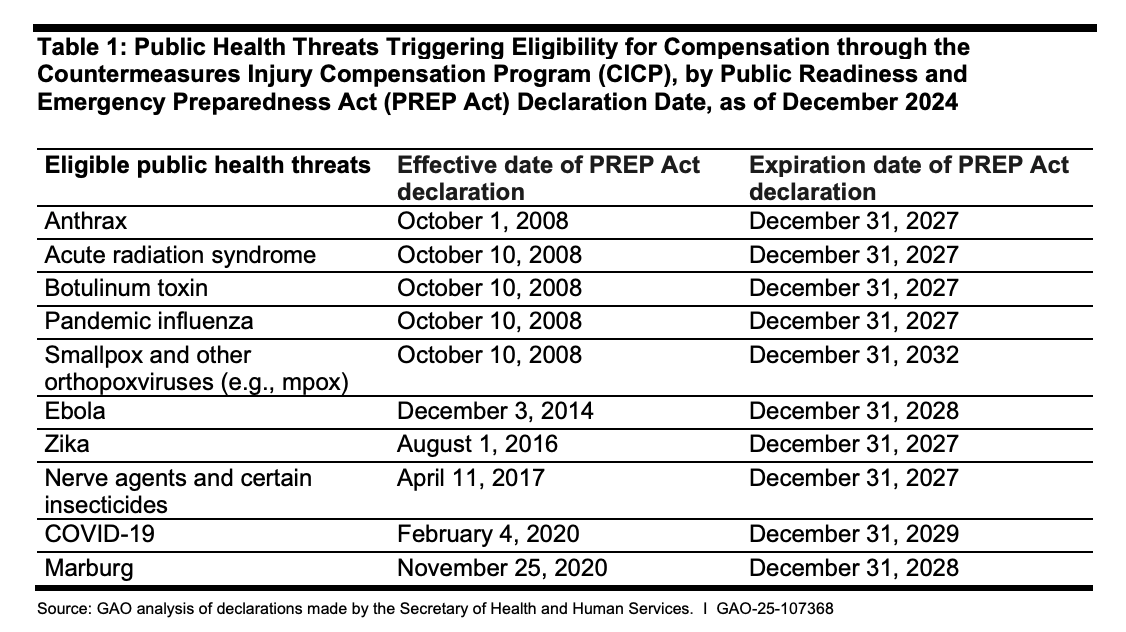
PREP ACT EMERGENCY DECLARATIONS IN EFFECT NOW
According to a December 18, 2024, report from the Government Accountability Office (GAO), these are the PREP Act emergency declarations that are in effect now. This means the HHS Secretary has determined that there is a current or future risk of a public health emergency that justifies indemnifying from any legal liability anyone who manufactures, distributes, administers or does anything else related to “countermeasures” (which the Secretary also determines) against these threats.
The arbitrary nature of these declarations raises so many questions, including:
None of these public health threats are actually posing an emergency now, so how does the Secretary know that the risk for anthrax, acute radiation syndrome, botulinum toxin, pandemic influenza, and Zika will extend until the last day of 2027, while the risk for Ebola and Marburg will continue through 2028, and COVID-19 will remain a public health emergency until the end of 2029, with monkeypox posing a mortal danger until 2032?
How can the Secretary justify giving legal immunity to anyone who does anything with any “countermeasures” for all of these future projected public health emergency risks?
Why has nobody challenged the Constitutionality of giving one public health official the power to declare emergencies that give blanket legal shields that are exempt from judicial review, require no Congressional oversight, and pre-empt any State law?
THE PREP ACT AND COVID-19
On March 17, 2020, Secretary of HHS Alex Azar issued the first “Declaration Under the PREP Act for Medical Countermeasures Against COVID-19,” and immediately post-dated it to February 4, 2020 (the date on which pharmaceutical companies were notified by the Pentagon that the novel coronavirus was a “national security threat”).
According to the Declaration, PREP Act immunity covers “any antiviral, any other drug, any biologic, any diagnostic, any other device, or any vaccine, used to treat, diagnose, cure, prevent, or mitigate COVID-19, or the transmission of SARS-CoV-2 or a virus mutating therefrom, or any device used in the administration of any such product, and all components and constituent materials of any such product.”
Immunity from liability is granted to “manufacturers,” “distributors,” “program planners,” “qualified persons,” and their officials, agents, and employees and any person authorized … to prescribe, administer, deliver, distribute or dispense the Covered Countermeasures, and their officials, agents, employees, contractors and volunteers.
The Declaration was amended/extended 12 times, the last amendment extending the Declaration until December 31, 2029.
This means that for 10 years, from the beginning of 2020 to the end of 2029, anyone who does anything related to any product designated as a “countermeasure against COVID-19” has no legal liability for any harm or damage caused by that product.
The Covid mRNA vaccines, developed and produced under Emergency Use Authorization and non-contractual military transaction instruments, with no legally binding regulatory standards or oversight, are of course included in the PREP Act coverage.
Thus, when Covid mRNA vaccine manufacturers documented thousands of deaths and injuries potentially caused by their products as early as February 2021, they knew they would be shielded from any liability. Indeed, as discussed at length in many Substack posts by Sasha Latypova, only one court case so far has managed to pierce the PREP Act liability shield. All others have failed, including Pfizer whistleblower Brook Jackson’s case, in which the government actually intervened to protect the pharmaceutical company against the complaint.
It turns out Joe Biden – under whose Administration the PREP Act was extended eleven times – was correct when he noted that under this law:
“A drug maker can be grossly negligent in making or distributing a drug, and still escape liability. It can even make that drug with wanton recklessness and escape scott-free after harming thousands of people.”
And Senator Ted Kennedy, whose nephew has had the sole authority to end the Covid PREP Act emergency declaration from the moment he stepped into his role as HHS Secretary, was correct is his damning assessment:
“This is a sham. There is no possibility of liability here.”
TAKE ACTION
If, after learning about the PREP Act, readers would like to take action, several initiatives are underway:
Join Sasha Latypova in asking HHS Secretary Kennedy to terminate the PREP Act emergency declaration for Covid.
Sign the petition put together by James Roguski to Repeal the PREP Act.
ACKNOWLEDGEMENTS
Much of the information in this article is based on the work of independent researcher Katherine Watt. I am grateful for her tireless investigations and meticulous documentation of the legal framework behind pandemic planning in general, and Covid in particular.



This is disturbing as hell.
Thank you Debbie for identifying again where the real meat on the bones resides.
I do have one question, that I do not think is covered in the post ... who specifically inserted the PREP Act at the last minute? Wouldn't that be instructive to know who's hands wrote it in?
Did I miss that bit?
Isn't it interesting that a lot of crooked congresspeople voiced dissent against this law?
It was like they did it to pretend like they were not on board with it. But then Biden is there doing it even though he opposed it?
Absurdity